home contact company.overview news sitemap print
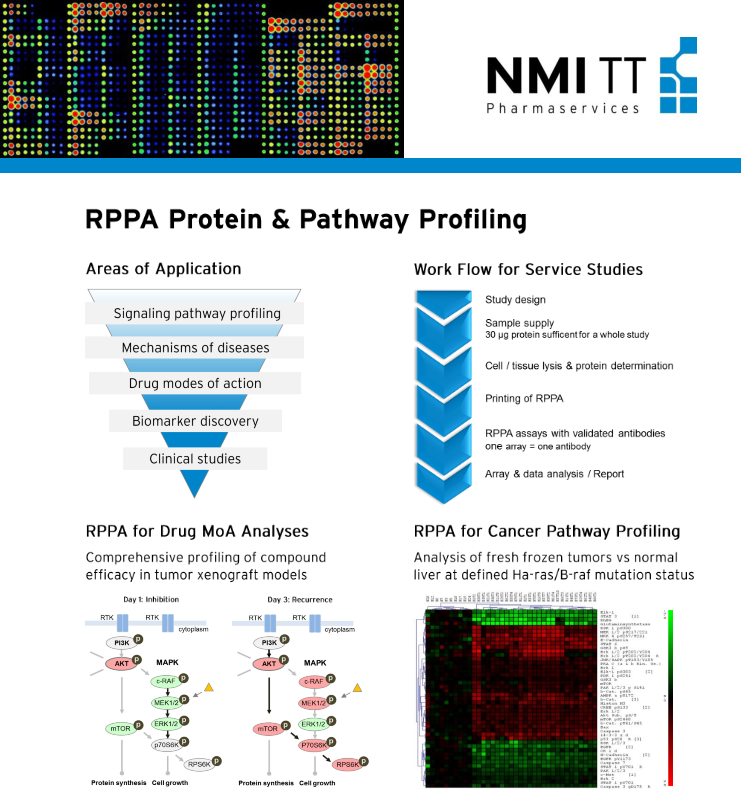
RPPA Signaling Protein & Pathway Profiling
- Preclinical and clinical sensitivity testing of drug candidates
- Investigation & evaluation of compound action on a molecular basis
- Key features
- Established technology, with proven added value
-
ISO9001 compliant, robust Standard Operating Procedures (SOPs)
-
More than 10 years of experience in designing and performing RPPA studies for life science industry and academic research
-
Study size: typically 20-160 samples, scalable up to 480 samples
-
Scope: up to 382 currently available pathway markers (total proteins and post-translationally modified forms), to be selected from our validated RPPA antibody list
- We also validate additional and/or our clients' own antibodies for signaling proteins of interest
- Established applications
-
Lead compound mechanism-of-action and translational studies
-
Quantification of on/off target & downstream signaling effects
-
Transient selective pathway inhibition and cross-pathway effects
-
Biomarker screening/verification of preclinical & clinical samples
-
Mechanisms and markers for treatment efficacy and resistance
-
Providing rationale for treatment combination strategies to overcome resistance mechanisms
- Typcial samples
-
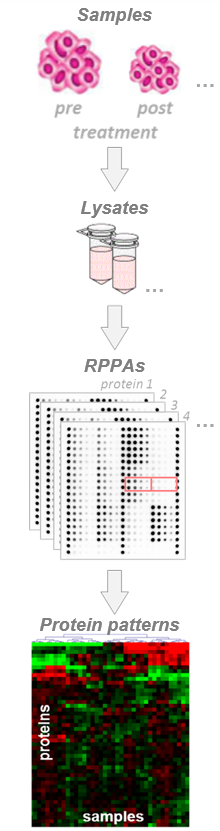
Cell cultures (2D; 3D, e.g. spheroids, microtissues)
-
Animal tissues (different organs)
-
Xenografts, PDX tumor models
-
Patient tissues, tumor biopsies, either fresh frozen (FF) or formalin-fixed & paraffin-embedded (FFPE)
- Minimum sample amount required for a typical RPPA study: 30 µg total protein at 3 µg/µl protein concentration
References
Pawlak et al. (2002) Proteomics
Saturno et al. (2007) Toxicologic Pathology
Pirnia et al. (2009) Proteomics
Assadi et al. (2013) Mol Cell Proteomics
Akbani et al. (2014) Mol Cell Proteomics
Tegnebratt et al. (2014) EJNMMI Research
Bader et al. (2015) Mol Cell Proteomics

 +49 7121 51530 13
+49 7121 51530 13