
home contact company.overview news sitemap print
Contact |
|
Dr. Oliver Pötz |
|

MS-based TXP Immunoassays
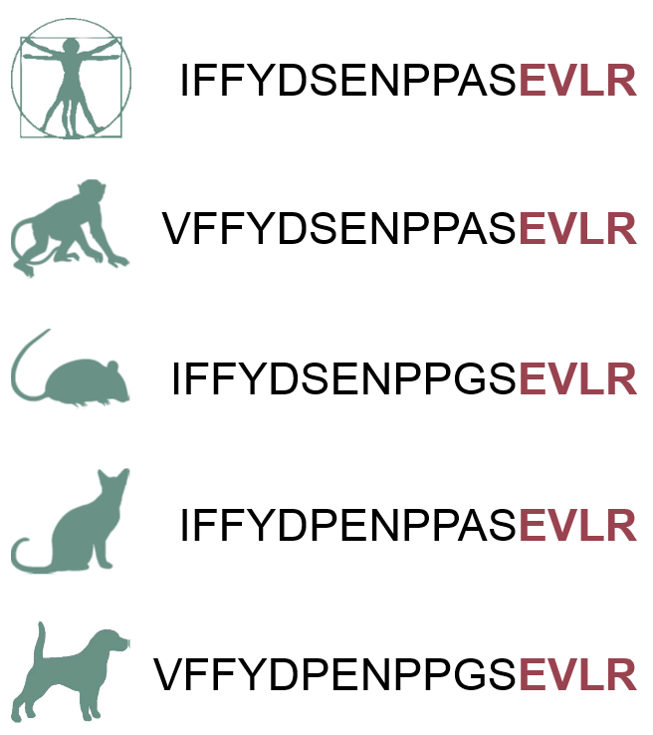
Mass spectrometry (MS) is a highly specific mean for the detection and quantification of proteins. The incorporation of a targeted enrichment step using antibodies led to further improvements in sensitivity and the development of mass spectrometry-based immunoassays. Here, the analyte - the protein itself or a tryptic peptide thereof - is enriched by an antibody prior to the mass spectrometric read-out.
MS-based immunoassays provide advantages over classical sandwich immunoassays, since the mass spectrometric readout confirms the identity of the analyte unambiguously and only one capture molecule is required. Additionally, the mass spectrometric data allow discrimination between different protein isoforms associated with related or alternatively processed or spliced genes, which is difficult when antibodies are used exclusively.
The lack of appropriate antibodies has so far limited the application of MS-based immunoassays approaches for protein quantification. Our novel concept of using group-specific anti-peptide antibodies, so-called Triple X Proteomics (TXP) antibodies for the enrichment of groups of signature peptides, enables us to develop multi-specific antibodies, which can be used for the enrichment step of multiple analytes harboring a common motif. Since the motifs comprise only 4 amino acids, TXP antibodies can be used for enrichment of protein families and for development of cross-species assays.
We have established such MS-based immunoassays employing TXP antibodies for several applications, including a number of cross-species ADME/Tox assays that represent truly translational marker assays 1-7.
- We offer assays for
-
Membrane protein quantification (GPCRs)
-
Coagulation factors
-
Cytochrome P450 (CYP) and drug transporter quantification
References
(1) Bauer M, Chicca A, Tamborrini M, Eisen D, Lerner R, Lutz B, Poetz O, Pluschke G, Gertsch J. Journal of Biological Chemistry 2012.
(2) Eisen D, Planatscher H, Hardie DB, Kraushaar U, Pynn CJ, Stoll D, Borchers C, Joos TO, Poetz O. Journal of Proteomics 2013.
(3) Hoeppe S, Schreiber TD, Planatscher H, Zell A, Templin MF, Stoll D, Joos TO, Poetz O. Molecular & Cellular Proteomics 2010.
(4) Poetz O, Hoeppe S, Templin MF, Stoll D, Joos TO. Proteomics 2009, 9, 1518-1523.
(5) Schneider S, Schreiber TD, Eisen D, Wiese C, Planatscher H, Pynn CJ, Stoll D, Templin M, Joos TO, Poetz O. Molecular & Cellular Proteomics 2012.
(6) Weiss F, Schnabel A, Planatscher H, van den Berg BH, Serschnitzki B, Nuessler AK, Thasler WE, Weiss TS, Reuss M, Stoll D, Templin MF, Joos TO, Marcus K, Poetz O. Scientific Reports 2015, 5, 8759.
(7) Weiss F, van den Berg BH, Planatscher H, Pynn CJ, Joos TO, Poetz O. Biochimica et Biophysica Acta 2013.

 +49 7121 51530 802
+49 7121 51530 802